The polarisation processes of charge shift, until the equilibrium state of dielectrics, are followed by shift current (polarisation currents). Shift currents of elastic bounded charges are momentary. Shift currents of any kind of slow polarisation are called absorption currents. Existence of free charges in a dielectric results in cross-cutting conductivity. So total current density in dielectric, called leakage current, is Jl = Ja + Jc.
Shift current density is determined by electrical shift vector D:
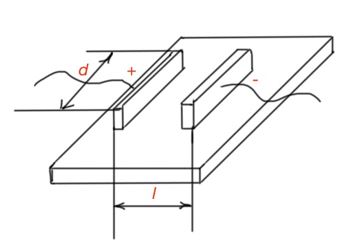
Dielectric conductivity can be considered by cross-cutting current, which is followed by the appearance of charges on the electrodes. When voltage is variable, conductivity is determined by cross-cutting current and polarisation currents. Dielectrics are characterised by ionic polarisation in most cases. Capacitor resistance between electrodes is considered by the formula:
Solid dielectrics are characterised by surface volume conductivity, bulk resistance and surface resistance.
Surface resistance can be determined by the formula:
This way we can determine bulk and surface conductivities:
Total dielectric conductivity Gtot is a sum of bulk and surface conductivities, corresponding to the total capacitor resistance Rtot. Time constant of a capacitor characterises the capacitor and can be determined by the formula τ0 = RtotC.
Gases are characterised by small electric conductivity in a ‘small’ electric field. Current can occur in gases only with ions or free electrons. Gas molecule ionisation may happen because of external factors, or as the result of strike ionisation. External factors, provoking ionisation are X-rays, UV-rays, radiation, thermal exposure and some others. During the ionisation process, positively and negatively charged particles occurs in gas. At the same time, positive and negative particles combine, and this process is called recombination. This process hinders the endless process of ion generation. So there are always a limited quantity of ions in gas.
Conductance of liquid dielectrics is related to liquid molecule structure. Polarisation of non-polar liquids is related to dissociated impurities. For polar liquids it is also related to self-dissociation. Current in liquids can be provoked by ions, as they can by big colloid particles.
Conductivity of solid dielectrics is related to movement of ions of the dielectric, of impurity ions, and sometimes by electron movement. The type of conductivity can be considered experimentally using Faradei Law. Ionic conductivity is related to the movement of a compound on the electrodes, which does not happen at electron conductivity. Solid dielectrics with ionic lattice conductivity is considered by ion movement, torn out from the lattice. In dielectrics with atomic or molecular lattice, conductivity depends on the impurities.
For ionic conductivity ion concentration depends on the temperature:
Mobility of the ions can also be represented by the exponent:
So dielectric conductivity has the following form:
If the current in dielectric is related to movement of different ions, then dielectric conductivity has the following form:
At low temperatures conductivity is related to ionised impurities – at high temperatures conductivity intrinsic.
Surface conductivity of a dielectric is caused by humidity and structural defects of the dielectric. Surface conductivity is small if dielectric surface is clean. Contamination of a dielectric slightly affects the conductivity of hydrophobic dielectrics, and deeply affects conductivity of hydrophilic dielectrics.
Hydrophobic dielectrics are non-polar, hydrophilic dielectrics are polar and ionic. The biggest increase of conductivity occurs with polar dielectrics that can be partially dissolved with water, with the appearance of electrolyte film on its surface. Impurities can stick to the polar dielectric surface, which also increases its conductivity. Poured materials also have quite large conductivity as they absorb water. Different methods of surface cleaning are used to decrease surface conductivity of a dielectric.