This post answers the question “What are defects in crystals?”. There are no naturally occurring ideal crystals. Every solid compound has some deviation from an ideal periodical lattice. These deviations are called defects or imperfections of the solid structure. They tentatively can be structured as dynamic and static. Dynamic defects can happen during mechanical, thermal or magnetic impact. The most frequent example of dynamic defect is phonons – temporary distortion of a crystalline lattice caused by thermal atom motion.
Among the static defects, we can point out point or atomic defects and linear defects. Atomic defects are vacancies and interstitials, impurities, foreign atom integration to the crystalline lattice. Linear defects are dislocations, pores, cracks and grain boundaries.
Imperfection concentration in the solid structure can be small, but their effect on the physical properties of the compound can be significant. For example, 1,000th of a percent of defects in the semiconductor crystal changes its conductivity 103 – 104 times. Linear defects make significant impact on mechanical properties of solid structures.
Before introducing the concepts of defects, we must introduce some basic concepts for materials. As has been said before, pure material with only one type of atom doesn’t exist. There are always some impurities which form defects in a solid. The most frequent metal is alloy. Alloy is a metal where impurities were intentionally added to modify some properties of the solid. A solid solution is a result of adding impurities into a metal. Solvent is an element which is present in a compound in the greatest amount. A Solute denotes an element in a minor concentration in a compound. A Solid solution is a compound which is formed by adding a solute atom to the solvent without changing a lattice. A Solid solution is homogenous, and impurity atoms are randomly dispersed across the compound. There are two types of point defect in solid solutions: substitutional and interstitial.
Vacancies and self-interstitials
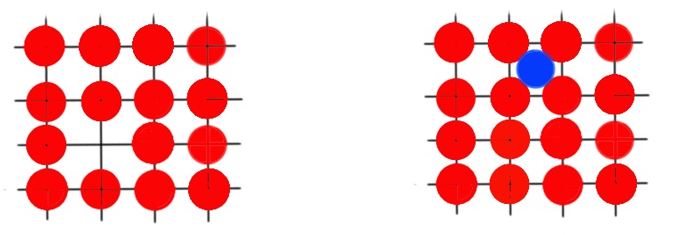
Vacancy is the simplest form of defect. All lattice cells are normally occupied, but in some places atoms are missing – these non-occupied sites are called vacancies. All solids contain vacancies and it is not possible to create a solid compound free of such defects. Vacancies are increasing entropy of a crystal. And the total number of vacancies , for a given quantity of material depends on the temperature by the Boltzmann’s equation:
where is a total number of atomic sites, is an energy needed for vacancy formation, is a Boltzmann’s constant.
This equation means that the number of vacancies increases exponentially with temperature. A self-interstitial is an atom in crystal placed into an interstitial site. For metals this kind of defect is quite rare, because the size of the atom of the metal is much bigger then interstitials, and it causes bigger deformations of the crystalline lattice. Types of vacancies and self-interstitials are presented below.
Dislocations
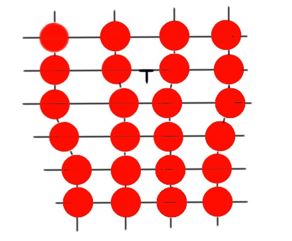
A dislocation is a linear type of defect around which some of the atoms are misaligned. Dislocations can also be edge type, when the extra portion of the atoms are on the edge of the crystal. As far as this is a linear defect, it is also called dislocation line. There is always lattice distortion around the dislocation line. The magnitude of the distortion decreases as it moves away from the dislocation line.
Another type of dislocation is screw dislocation or shear dislocations. This type of dislocation is formed because of the shift of a part of the lattice relative to another part. You can see both types of dislocations in the Figure 3.
Most dislocations in the crystalline compound are neither line, nor screw. These dislocations are called mixed dislocations. Every solid compound has dislocations, caused by mechanical stresses and heat treatment.
Figure 3 shows dislocations in the alloy.
External surfaces
An external surface is the most common interfacial defect. This external surface is where the compound terminates. Surface atoms are not bonded to the maximum number of neighbour atoms. So the energy of these atoms is bigger than the energy of all the rest of the atoms. And as we know, every system tends to minimise its energy. And these external atoms also tend to minimise their energy.
Grain boundaries
Another example of interfacial defect is a grain boundary. This is the boundary between two or more grains in a polycrystalline material. There are some atomic mismatches on the grain boundary region, which is about several atoms wide. And the lattices of grains can be different. Not all the atoms on the boundaries are bonded irregularly, this means that the boundary energy is growing. The magnitude of this energy is a function of the angle of misorientation of the boundary atoms – bigger angle, bigger boundary energy. Boundary energy makes grain boundaries more chemically reactive than grains.
Bulk defects
Bulk defects are those formed during compound production. The bulk defects include cracks, pores, foreign inclusions and other defects. They are all the result of fabrication process. Some of these defects impact the compound properties, even more significantly than the ones described in this module.Atomic vibrations
Every atom oscillates solidly around its position in a lattice. And this atomic behaviour is also a defect called atomic oscillation or vibration defects. Each oscillating atom has different oscillation frequency, amplitude and energy. At a given temperature, there is a distribution of oscillation energies around average energy magnitude. The oscillation energy of every atom varies at a random manner. And oscillation energy increases with increasing temperature. Many properties and processes in solids depend on the atom’s vibration energy.
More education posts are available at our Reddit community r/ElectronicsEasy.